Copyright © 2017 DR.NOAH BIOTECH All rights reserved.
Email. contact@drnoahbiotech.com | Tel. +82.31.546.1519 | Fax. +82.31.546.1529
(16229) 경기도 수원시 영통구 창룡대로 256번길 91, 에이스광교타워2 1208호
DR.NOAH BIOTECH
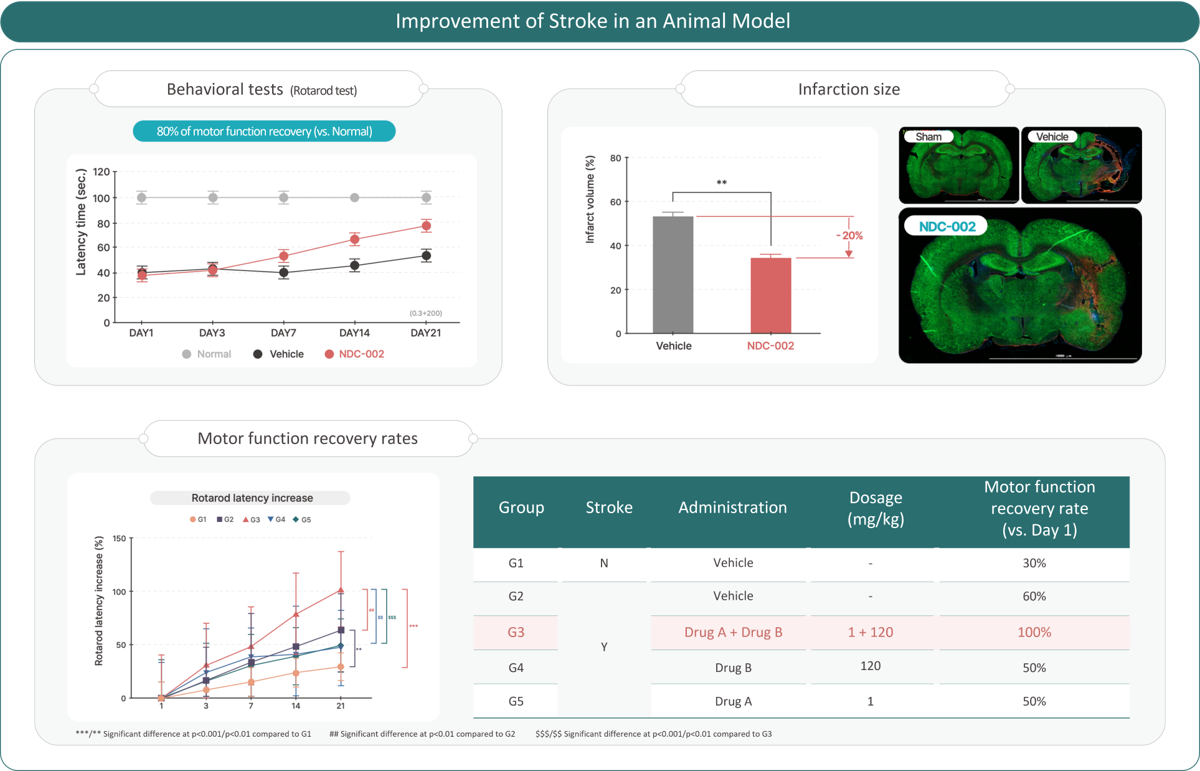
NDC-002 Stroke Recovery
Stroke remains a leading cause of death and disability worldwide,
yet there are no approved therapies for long-term functional recovery after acute treatment.
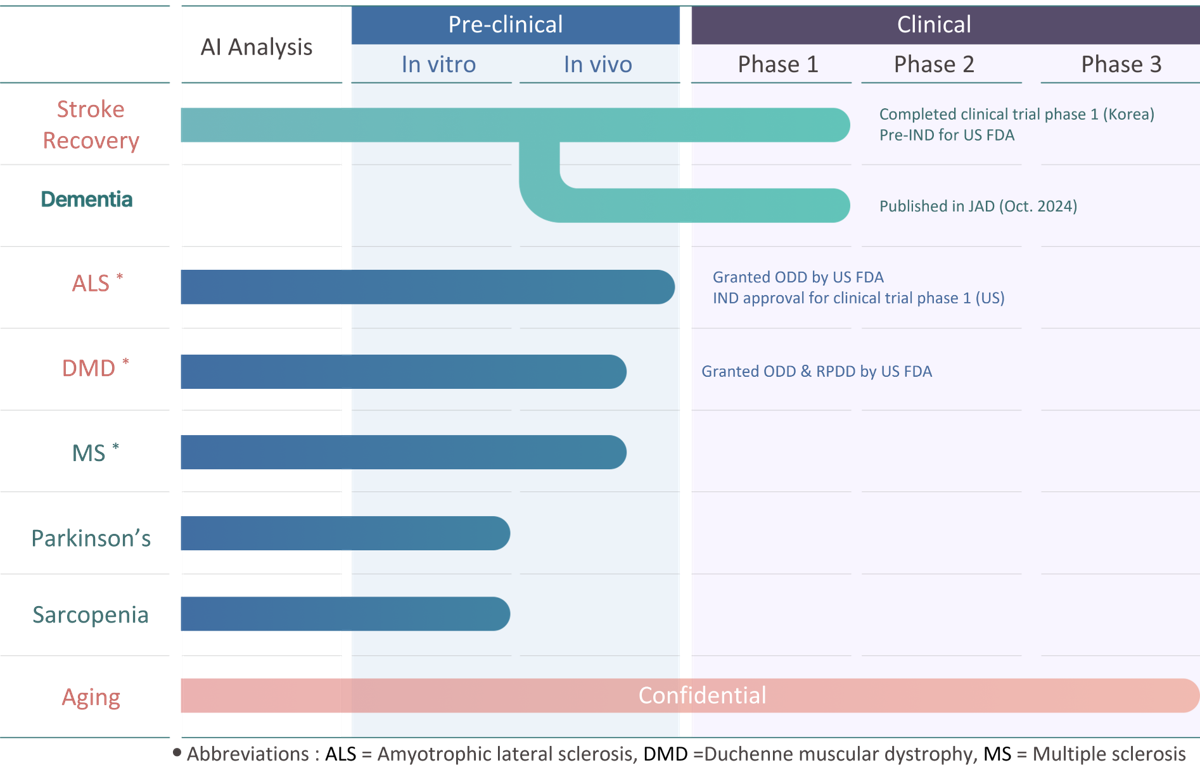
NDC-002, discovered via DR.NOAH’s ARK platform, is the first AI-driven New Drug Combination for post-stroke recovery.
Combining neuroprotection, neurogenesis, and anti-inflammation, it achieved 80% motor recovery in preclinical trials and became the first AI drug to complete Phase 1 in Korea (2023),
addressing a long-standing unmet clinical need.
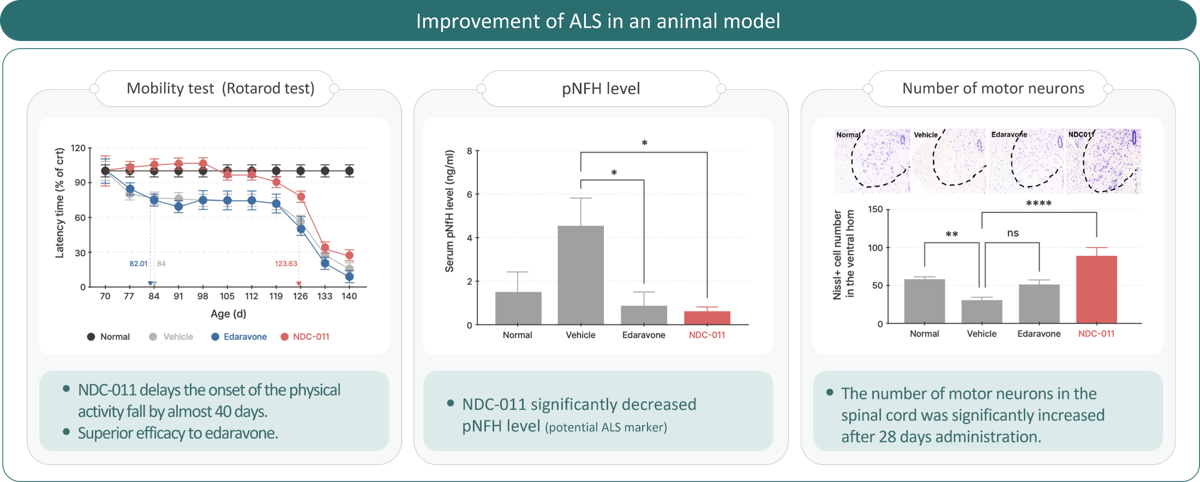
NDC-011 ALS
Amyotrophic Lateral Sclerosis (ALS) is a fatal neurodegenerative disease with no cure and poor prognosis,
with an average survival of only 2 to 5 years due to progressive motor neuron loss.
NDC-011, developed through AI-based drug discovery, is a New Drug Combination that uniquely inhibits motor neuron loss and promotes new neuron generation.
It was granted Orphan Drug Designation (ODD) by the U.S. Food and Drug Administration (FDA) in 2023 and received Investigational New Drug (IND) approval for a Phase 1 clinical trial in 2024.
In preclinical models, NDC-011 demonstrated superior efficacy compared to Edaravone, offering multi-target benefits to improve patient outcomes.
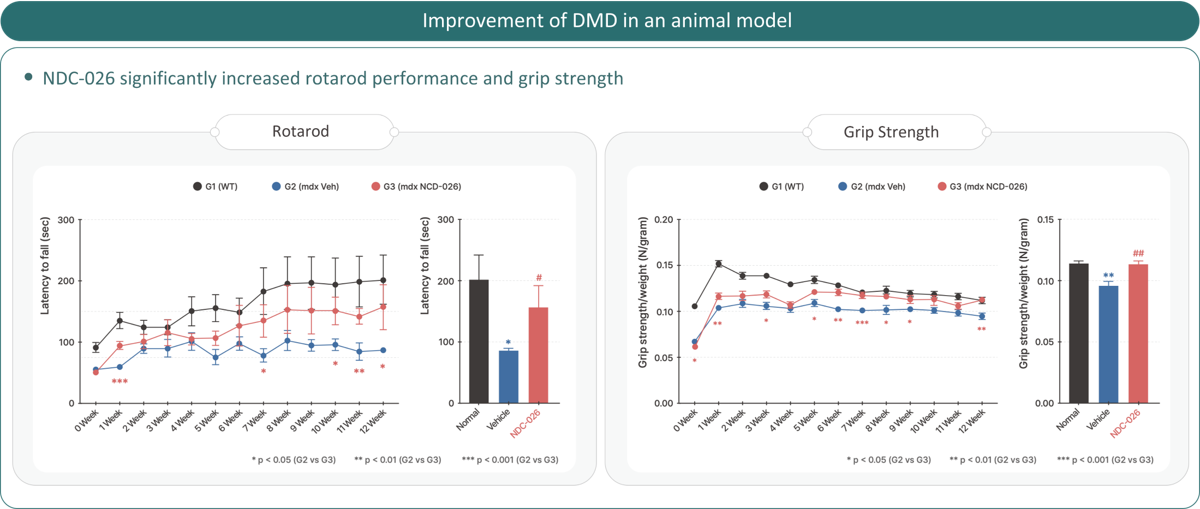
NDC-026 DMD
Duchenne Muscular Dystrophy (DMD) is a progressive genetic muscle disorder affecting boys,
with limited treatment options and poor prognosis. NDC-026, designed via AI discovery, is a New Drug Combination that works through multi-target mechanisms, enhancing muscle differentiation and regeneration while preventing atrophy.
It has received both Orphan Drug Designation (ODD) and Rare Pediatric Disease Designation (RPDD) from the US FDA. With proven efficacy in preclinical functional recovery tests, NDC-026 offers broad applicability to all DMD patients, representing a promising new treatment option.