
Doctor Noah Biotech (CEO Lee Ji-hyun, Doctor Noah) announced on the 11th that the non-clinical study results of the donepezil combination drug 'NDC-060' were published in the international academic journal Journal of Alzheimer's Disease. The paper published this time is a paper that reveals the efficacy and mechanism of NDC-060, a combination drug that can improve the side effects caused by donepezil.
Donepezil is the first drug approved by the U.S. Food and Drug Administration (FDA) for the treatment of Alzheimer's Dementia (AD), and helps improve cognitive function in dementia patients. It is the most commonly prescribed drug for treating mild or moderate dementia, and has recently been prescribed for mild cognitive impairment (MCI).
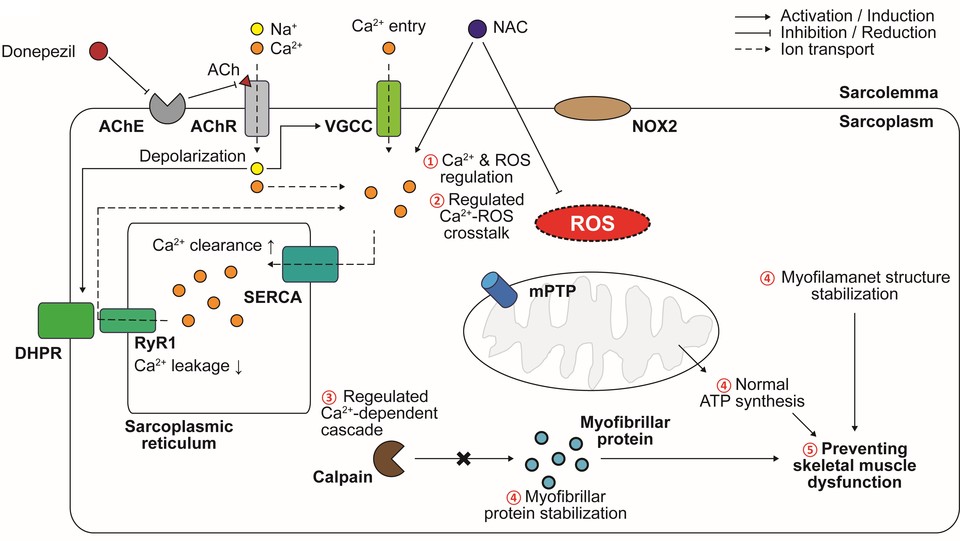
Donepezil acts on the central nervous system to alleviate dementia symptoms, but it can also cause various side effects if it acts on the peripheral nervous system. Representative symptoms include nausea, diarrhea, insomnia, vomiting, muscle cramps, fatigue, and loss of appetite.
Dr. Noah discovered a drug that improves the side effects of donepezil, an important drug in the treatment of dementia, through the artificial intelligence (AI) platform ARK, and developed the donepezil combination drug NDC-060. According to the company, NDC-060 improved muscle-related side effects such as decreased spontaneous movement, decreased muscle tone, and tremors in nonclinical animal experiments.
Dr. Noah is an AI new drug development company that develops treatments for nervous and muscular diseases. Recently, NDC-011, a treatment for Lou Gehrig's disease (ALS) discovered using its own AI technology, received FDA approval for a phase 1 clinical trial plan (IND) and is preparing for a global clinical trial next year.
Additionally, NDC-026, a treatment for Duchenne muscular dystrophy (DMD), received Rare Pediatric Disease Designation (RPDD) and Orphan Drug Designation (ODD) from the FDA in April.
Dr. Lee Ji-hyun, CEO of Dr. Noah Biotech, said, “I believe that developing drugs that can help patients who are having difficulties due to side effects of widely used drugs is as meaningful as developing new drugs,” adding, “We will continue to think about this and develop drugs in the future.”
dynam@hitnews.co.kr
Reporter Nam Dae-yeol Entered 2024.11.11 08:00 Comments 0


