[Interview of Bio CEO] CEO Jihyun Lee of DR.NOAH BIOTECH dreams of developing 'blockbuster'--going beyond the technology transfer of six pipelines

[Medigate News Reporter Minji Seo] Neurological disorder is one of the complex disorders that are difficult to treat by controlling a single target. It is composed of complex mechanisms connected organically. A meaningful effect is achieved only by setting multiple targets.
Considering such characteristics of the disorder, DR. NOAH BIOTECH is developing treatment with a strategy called 'combinatory drug.' The company is actively making use of the artificial intelligence (AI) platform developed to increase the efficiency of R&D pipelines.
In addition to reduced time and increased efficiency, its benefit also includes having no controversy over privacy protection, accuracy, and standardization resulted from adopting the company's unique method of data collection, analysis, and application
Together with CEO Jihyun Lee of DR, NOAH BIOTECH, Medigate News discussed the AI platform, the features of dew drugs being developed, the constructed pipeline, and the future direction and plans of R&D.
DR.NOAH BIOTECH is a bio-venture constructing pipeline with a focus on neurological disorders. Moving further from the development of new drugs, the company is also an AI development venture. The company collects and analyzes data on its own to construct an AI system for clinical trials.
Owing to that, DR. NOAH BIOTECH is currently divided into Research Center and Development Center. The Research Center is composed of ▲Data Management Team, in charge of the whole dielectric substances and chemical compounds used for artificial intelligence; and the management of internal experiment results and literature data, ▲Data Analysis Team in charge of drug predictions and the development of all analytical software of the artificial intelligence on the ARK platform developed by the company and ▲Validation Team in charge of drug development strategy planning, the validation of medicine's effects on cells and animals, and the creation of experimental data to be used for artificial intelligence learning.
The Development Center has ▲Business Development Team in charge of deciding internal development pipeline, planning IP (Intellectual Property) strategies, and implementing joint development with external organizations ▲RA Team to plan and conduct licensed clinical trials and manage the entire product development cycles. ▲ Formulation/Dosage Form Team, in charge of developing the formulation and dosage forms of drugs.
The six R&D pipelines of DR. NOAH BIOTECH... everything is 'combinatory drugs'
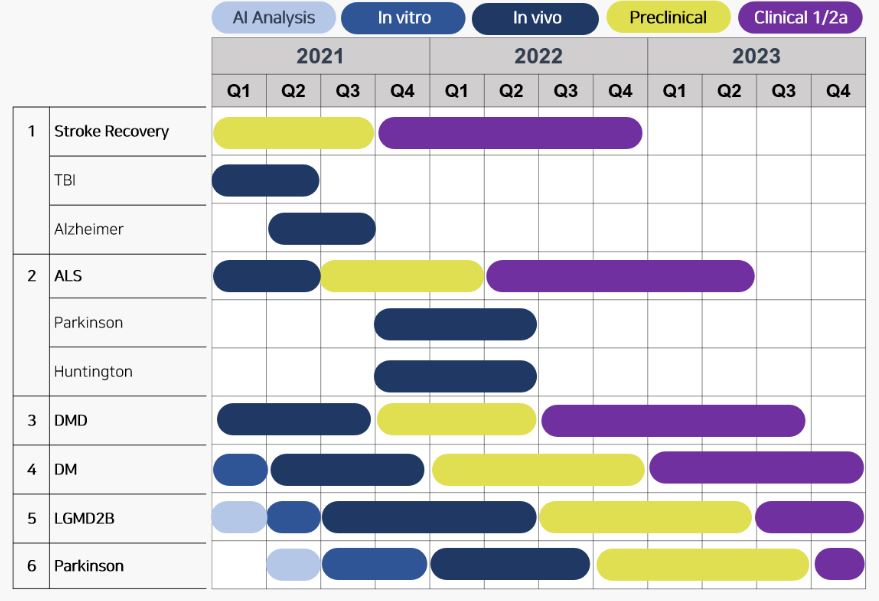
Based on its research infrastructure, DR. NOAH BIOTECH currently owns six R&D pipelines that all target neurological disorders. Another characteristic of these is that the new drugs are all 'combinatory drugs.'
CEO Lee said that 'if you look at the neurological drugs being developed, they are developed to prevent the destruction as they focus on the break-down of the neurons" "But neurological disorders are composed of very complex mechanisms connected organically. Thus, controlling a single target is not likely to yield a clinically meaningful effect."
CEO Lee also mentioned that "I thought that setting multiple targets would yield meaningful results. So, currently, the company is developing new drugs based on combinatory drug strategy." "We are designing combinatory drugs that can suppress destruction and also create more neurons. Thus, we are developing a new drug that does not only treat the disorder but also 'recover' from the disorder."
In fact, the most popular pipeline of DR. NOAH, the Stroke Recovery, is also being developed with the focus on assisting recovery and penetrating the thrombus.
CEO Lee said, "the existing stroke medicines only aim to remove blocked thrombus in 6 hours. But people don't usually remove them within 6 hours at the hospital. People usually have slow recovery due to the delayed treatment." She further explained that "We are developing a drug that prevents neuroinflammation and restores the brain from swelling, inflammation, or destruction of brain cells; and another drug for differentiation and production of neurons. And these two will be put into a single capsule." This is based on the principle that treatment for inflammation and restoration of brain cells work together in the form of the combinatory drug for faster recovery."
Above mentioned new drug aims for technology transfer or commercialization in between 2022~2023. The company plans to apply for the investigational new drug (IND) in July this year.
Aside from the recovery treatment for stroke, the company also has pipelines on rare neurological disorders such as Amyotrophic Lateral Sclerosis (ALS), Duchenne Muscular Dystrophy (DMD), Myotonic Dystrophy, and Limb-girdle Muscular Dystrophy Type 2B(LGMB2B). The company plans to start clinical trials on ALS and DMD pipelines.
CEO Lee also revealed that "ALS damages the motor neuron. Likely, a combinatory drug is needed to prevent the destruction of motor neurons and promote the production simultaneously. DMD is also a disorder causing muscle problems. So, we are researching with a focus to develop a combinatory drug to reduce muscle death and to facilitate muscle formation."
An artificial intelligence platform developed by the company achieved faster clinical trials
The pipelines make use of the ARK platform. It is an artificial intelligence system the company developed on its own to strengthen, to increase efficiency, and to save time,
ARK platform is predicted to spend 2~2.5 years before the clinical trials after the start of the project. At the same time, only 1/3 of the development cost is forecasted to be required.
She also introduced that the most significant difference from the other new artificial intelligence drug-developing companies lies in the artificial intelligence analytical program called 'NeuroRG' embedded within the ARK platform.
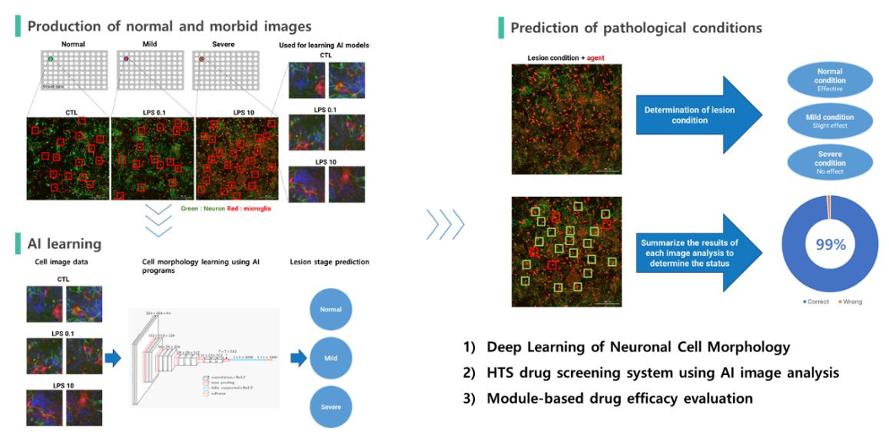
CEO Lee said, "After NeuroRG processes 10,000 compounds using HTS equipment available at the company's laboratory, the system evaluates the drug's effectiveness within two weeks using image analysis of the neurons." "No information of the compound is required in the process. The system still works on compounds with missing or no information. Besides, the technology is considered outstanding as the millions of cell images used in constructing the artificial intelligence model are internally created."
CEO Lee presented, "The local artificial intelligence companies are often attacked with the data ownership by the investors. As we manufacture our own and use owned artificial intelligence model, we are free from such comment." "Having a system running automatic 24-hour drug's effectiveness verification experiment is another strength too."
A regular screening experiment for accurate validation of efficiency is being conducted using an in-house drug library that the company constructed. Consistent data production is possible from the massive cell production, drug preparation, drug processing, dying, preprocessing to cell image works through automatic division equipment and automatic imaging equipment.
Despite being a unique artificial intelligence new drug development company, the company will still focus on internal research and development rather than focusing on technology transfer or market listing. "We expect to have enough clinical data from one or two years from now. As we have completed system development, we are now at the stage to show results. We plan to acquire capital through series B investment at the end of this year and use the capital to strengthen the pipeline R&D. "Once certain results are released probably in 2023, we plan on executing an initial public offering (IPO) based on the agreements made with the investors", said CEO Lee.
Meanwhile, CEO Lee said that although the company may transfer the technology after developing new drugs for our company's growth, having partnerships with large pharmaceutical firms help her learn about the problem caused by a venture, such as the lack of experience.
CEO Lee said, "through joint research with pharmaceutical companies, I am learning the actual specification required in the pharmaceutical industry and other perspectives in terms of research. Also, this will help us save infra that is often depleted at the last stage of development. We are currently working on the level that our employees can handle." "For the joint research, in addition to drugs on neurological disorders, we are also working on drug development that our pharmaceutical partner company requested."
The company wishes to develop, sell, and even 'blockbuster-ize' the new drugs in the long run.
CEO Lee spoke of her aspiration as "People used to distrust the bio-industry. Now, the timing is good. Many funds are circulated, and the government supports with great interest" "Making a new drug only has a 1/1000~10000 possibility, but we will not stop on technology transfer. Our mid-to-long-term goal is to do commercialization on our own using the optimistic situation. We want to go beyond being a bio-venture and establish ourselves as a new pharmaceutical company model with constantly good sales results. Another prospective direction that I have is 'blockbuster-izing' the developed drugs.





